Despite more than 18% of the global population living in Africa, the continent typically hosts a relatively small percentage of global clinical trials compared to other regions.[1] Logistical challenges and regulatory complexities in trial supply management are often cited as reasons for this unrealised potential, but this is fast changing as we see pharma and biotechs increasingly choosing countries in Africa for their clinical trials.
The increase in clinical trials in Africa can be attributed to several factors. One of the key causative reasons is the high disease burden in the continent, including infectious diseases and non-communicable diseases, as well as high maternal and child mortality rates, malnutrition and inadequate healthcare infrastructures. This provides an opportunity for innovation and development in the region, making it a highly suitable location for pharmaceutical companies to trial new drugs. Additionally, the presence of a large genetically diverse population in Africa who are eager to participate in clinical trials further contributes to the growth of trials across the continent.
Why clinical trials need to diversify
The opportunity for more diverse clinical trials is essential for the continued improvements of healthcare for diverse ethnic groups, as well as furthering the understanding of diseases in general. A study by Varma and colleagues, reported in the British Medical Journal (BMJ) in January 2023, found that only one out of 25 pharmaceutical companies that sponsored FDA-approved novel oncology therapeutics between 2012 and 2017 included a sufficient number of minority groups in their clinical trials.[2]

Inclusion of Under-Represented Racial and Ethnic Groups in Cardiovascular Clinical Trials, published in the National Library of Medicine, stated that just half of 153 randomised cardiovascular clinical trials reported any racial or ethnic information, despite more calls for diversity and representation in cardiovascular studies. The paper also stated there had been no significant improvement in the inclusion of traditionally under-represented ethnic groups. This is a worrying omission, as mortality rates for heart disease and strokes tend to be higher for black adult patients. However, factors in driving disparities in mortality rates are still not fully understood.
Alongside providing patient recruitment opportunities from a wide range of ethnic backgrounds, clinical trials in Africa have been at the forefront of improving the representation of women’s health.
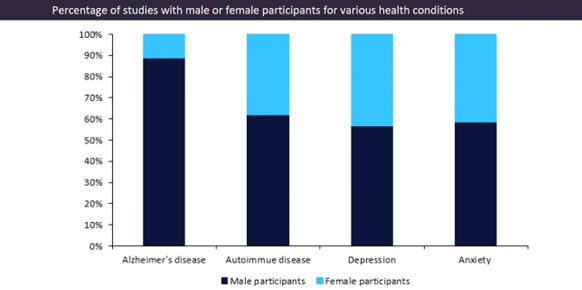
Historically, female participants have often been underrepresented in clinical trials as medical research has been more focused on male health. Furthermore, GlobalData’s clinical trials database has revealed that even for conditions that disproportionately affect women, the participant demographic in trials is heavily male.[3]
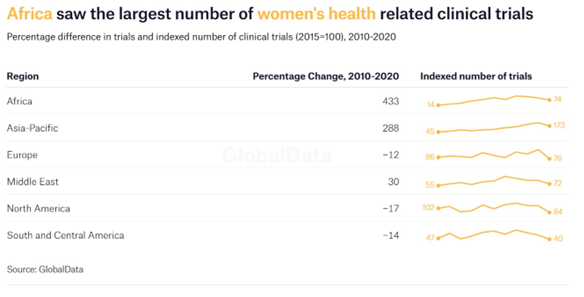
There are positive signs in Africa. The continent saw a 433% increase in the number of trials for women’s health taking place between 2010 and 2020, making it the region with the largest growth in this area. This substantial rise has elevated Africa to the fourth highest region worldwide in terms of the total number of trials taking place, up from sixth place in 2010.
Overcoming challenges for CROs in Africa
The operational costs in many African countries are competitive compared with more saturated markets, making Africa an appealing destination for pharmaceutical companies and contract research organisations (CROs). Furthermore, the regulatory environment is evolving, with African nations streamlining clinical trial approvals and adopting more transparent processes. This helps to reduce the bureaucracy that previously hindered the initiation and progress of significant medical research. Such improvements have not only attracted foreign investment, but have also fostered local research capabilities.
Kenya exemplifies Africa’s burgeoning role in clinical trials with its investments in medical research facilities and a growing workforce skilled in clinical practices.
Throughout sub-Saharan Africa, Oximio is a leading logistics and supply chain solutions provider specialising in the pharmaceutical and healthcare sectors. The company is playing a crucial role in overcoming the challenges traditionally faced by clinical trials in Africa and is providing a one-stop-shop for African clinical supplies via its local depot in South Africa and a bonded warehouse in Kenya.
The opening of Oximio Kenya in 2021 was a significant step in expanding operations and service coverage for the entire African continent and a key enabler in reshaping the clinical supply chain.
In the forthcoming webinar, Oximio will explain the major challenges behind this reshaping process – such as with infrastructure and regulations – and the strategy implemented by experts on the ground to overcome them. Meanwhile, analysis from GlobalData will cover the clinical trials market in sub-Saharan Africa and the key trends. Click here to learn more.
References:
[1] https://pharma1.globaldata.com/News/clinical-trials-in-africa-where-there-is-a-challenge-there-is-an-opportunity_198785
[2] https://pharma1.globaldata.com/News/bmj-finds-96-of-oncology-drug-developers-inadequately-include-minority-groups_171335
[3] https://pharma1.globaldata.com/News/women-are-underrepresented-in-trials-for-conditions-that-predominately-affect-them_182082



