
Lyophilization, also referred to as freeze-drying, is a complex process that removes water from the drug product to preserve the product’s chemical and physical integrity. In this blog, we will discuss the process of lyophilization cycle development and explore its challenges.
Understanding the lyophilization process
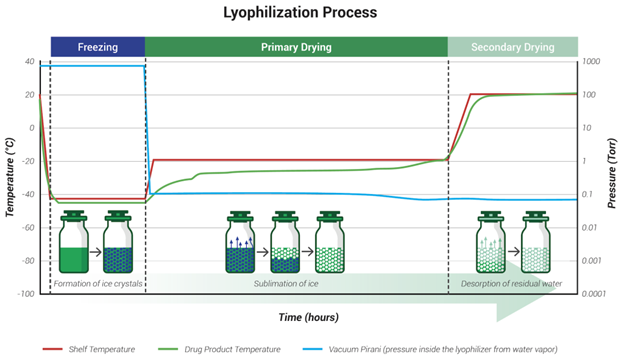
Lyophilization involves three primary phases: freezing, primary drying (sublimation), and secondary drying (desorption). The process begins by freezing the product to solidify the water content. During primary drying, a vacuum is pulled on the drug product and the temperature of the shelf is increased to sublimate frozen water in the drug product—converting ice directly to vapor and removing it via vacuum. Secondary drying then removes the residual moisture through desorption, usually with a slight increase in temperature. The result is a product with an extended shelf life, improved stability, and reduced susceptibility to degradation.
Advantages of lyophilization
An effective lyophilization cycle is ideal for two major purposes: product stability and ease of transportation.
Product stability: Many pharmaceuticals, vaccines, and biopharmaceutical products are sensitive in solution. Lyophilization removes water to prevent degradation, ensuring the product remains potent and safe for use.
Ease of transportation: Lyophilized drug products typically require non-frozen storage conditions, avoiding the requirements, complexities, and costs associated with cold-storage distribution.

Lyophilization cycle development process
The development of an optimal lyophilization cycle involves several steps:
Formulation design: The product’s formulation must be carefully designed to ensure compatibility with the lyophilization process. Excipients, such as cryoprotectants, are often added to protect the drug product during freezing and drying, and bulking agents are used to enhance cake quality.
Cycle design: The cycle’s parameters, including freezing rate, shelf temperature, and vacuum pressure, are determined based on the product’s characteristics and stability requirements. The CDMO will perform a thermal characterization of the drug product to establish a cycle design. This cycle is then fine-tuned through experimental trials.
Process optimization: Multiple cycles are tested to identify the one that best preserves the product’s quality while achieving efficient drying times. Water removal will be optimized and the lyophilized drug product will be tested in stability studies.
Validation: The selected cycle is then subjected to validation studies to ensure reproducibility, consistency, and robustness. This step is vital to meet regulatory standards. Once the cycle has been validated, the cycle will be transferred to an industrial lyophilizer.
Challenges in lyophilization cycle development
Developing a successful lyophilization cycle is not without its challenges:
Product variability: Different batches of the same product can exhibit varying responses to the lyophilization process, making cycle development complex. Also, the process may require tweaks depending on the lyophilizer, the volume of drug product in the lyophilizer, and the container that’s used.
Scale-up issues: Transferring a successful lab-scale cycle to larger production scales can lead to unexpected challenges due to differences in heat and mass transfer.
Regulatory compliance: Pharmaceuticals, especially aseptically filled parenteral drugs, are heavily regulated, necessitating meticulous documentation and validation to meet compliance standards.
Conclusion
Lyophilization offers several advantages. It ensures product stability, extends shelf life, and simplifies storage and distribution. The process of developing an effective lyophilization cycle is complex and requires significant knowledge, work, and a well-designed plan to execute. Despite its challenges, the successful development of a lyophilization cycle is essential for delivering safe, effective, and high-quality therapies to patients worldwide.
About Sharp’s services
Sharp is a leader in pharmaceutical packaging, clinical trial supply services and small-scale sterile manufacturing. For 70-plus years, we’ve provided solutions to pharma and biotech clients from Phase I trials through to commercial launch and lifecycle management. With facilities in the United States, United Kingdom, Belgium and the Netherlands and 30-plus clinical depots globally, covering every region of the world, our experience is your strength.



